TOP
 Events & Outreach
Events & Outreach
 News & Announcements
News & Announcements
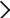 Electronic and coordination structures of metal porphyrin complexes in aqueous solutions probed by soft X-ray absorption spectroscopy
Electronic and coordination structures of metal porphyrin complexes in aqueous solutions probed by soft X-ray absorption spectroscopy
Electronic and coordination structures of metal porphyrin complexes in aqueous solutions probed by soft X-ray absorption spectroscopy
JapaneseResearchers in the Institute for Molecular Science and RIKEN and Nagoya University investigate metal‒ligand delocalization of metal porphyrin complexes in aqueous solutions, this study probes the electronic structures of both the metal and ligand sides using soft X-ray absorption spectroscopy (XAS) at the metal L2,3-edges and N K-edges, respectively.
In the N K-edge XAS spectra of the ligands, the C=N π* peaks of cobalt protoporphyrin IX (CoPPIX) show higher energy shifts than those of iron protoporphyrin IX (FePPIX), owing to the different electronic configurations and spin multiplicities of metal porphyrin complexes.
These spectra are useful for discussing the central-metal dependence of metal‒ligand delocalization. Meanwhile, the N K-edge XAS of CoPPIX and the inner-shell calculations of different hydration models reveal that CoPPIX maintains its five-coordination geometry in aqueous solution.
For details, please see the link below:
Electronic and coordination structures of metal porphyrin complexes in aqueous solutions probed by soft X-ray absorption spectroscopy![]() (Institute for Molecular Science)
(Institute for Molecular Science)
(Sep 17, 2024)
