12.2 REUS for the (Ala)3 in water
Contents
- Preparation
- 1. Setup
- 2. Minimization and pre-equilibration
- 3. Equilibration
- 4. Production run
- 5 Analysis
- 5.1 Make converted trajectory files for analysis
- 5.2 Sort coordinates in DCD files by parameters
- 5.3 Calculate the acceptance ratio
- 5.4 Plot the time courses of replica index and target distance
- 5.5 Analyze the end-to-end distance of (Ala)3
- 5.6 Free energy calculation using MBAR
- 5.7 Calculating PMF of distance distribution
- References
Efficient conformational sampling is one of the difficult issues in the computational biophysics. Since complex biomolecules have many local-minimum energy states, conventional MD simulations often get trapped there. Umbrella sampling method has been widely used to calculate free energy profile. In 2000, Sugita et al. proposed the replica-exchange umbrella sampling method (REUS [1], which is also called Hamiltonian REMD [2] or Window exchange umbrella sampling [3]), in which the restraint potentials are exchanged between replicas to enhance conformational sampling. In this tutorial, we demonstrate a REUS simulation of a small peptide using GENESIS.
Preparation
First of all, let’s download the tutorial file (tutorial22-12.2.zip). This tutorial consists of five steps: 1) system setup, 2) energy minimization and pre-equilibration, 3) equilibration, 4) production run, and 5) trajectory analysis. Control files for GENESIS are already included in the file. Since we use the CHARMM force field, we make a symbolic link to the CHARMM toppar directory (see Tutorial 2.1).
# Download the tutorial file
$ cd ~/GENESIS_Tutorials-2022/Works
$ mv ~/Download/tutorial22-12.2.zip ./
$ unzip tutorial22-12.2.zip
# Let's clean up the directory
$ mv tutorial22-12.2.zip TRASH
# Let's take a note
$ echo "tutorial-12.2: REUS simulation for ala3" >> README
# Make a symbolic link to the CHARMM toppar directory
$ cd tutorial-12.2
$ ln -s ../../Data/Parameters/toppar_c36_jul21 ./toppar
$ ln -s ../../Programs/genesis-2.0/bin ./
$ ls
1_setup 3_equilibrate 5_analysis toppar
2_minimize_pre-equi 4_production bin
1. Setup
In this tutorial, we simulate the (Ala)3 in water. We use the same input PDB and PSF files as in Tutorial 3.2.
# Prepare the input files
$ cd 1_setup
$ ln -s ../../tutorial-3.2/1_setup/3_solvate/wbox.pdb ./
$ ln -s ../../tutorial-3.2/1_setup/3_solvate/wbox.psf ./
2. Minimization and pre-equilibration
Similar to the conventional MD simulations, we carry out an energy minimization for the initial structure, followed by a short MD simulation to equilibrate the system. We use the same protocols described in Tutorial 3.2. Since we have already equilibrated the system in Tutorial 3.2, we use the restart file obtained previously.
# Take over the restart file obtained in Tutorial 3.2
$ cd ../2_minimize_pre-equi
$ ln -s ../../tutorial-3.2/3_equilibrate/eq3.rst
3. Equilibration
Final goal of this tutorial is to calculate the free energy profile (or potential of mean force) as a function of the end-to-end distance of the (Ala)3. We are going to use 14 replicas in the REUS simulation. Since each replica has an individual restraint potential, we have to equilibrate the system using 14 different restraints before the production run. Let’s take a look at the control file:
# change directory
$ cd ../3_equilibrate
# check the control file (only important sections are shown below)
$ less INP
[INPUT]
topfile = ../toppar/top_all36_prot.rtf # topology file
parfile = ../toppar/par_all36m_prot.prm # parameter file
strfile = ../toppar/toppar_water_ions.str # stream file
psffile = ../1_setup/wbox.psf # protein structure file
pdbfile = ../1_setup/wbox.pdb # PDB file
rstfile = ../2_minimize_pre-equi/eq3.rst # restart file
[OUTPUT]
logfile = eq_rep{}.log # log file of each replica
dcdfile = eq_rep{}.dcd # DCD trajectory file
remfile = eq_rep{}.rem # parameter index file
rstfile = eq_rep{}.rst # restart file
[REMD]
dimension = 1
exchange_period = 0
type1 = RESTRAINT
nreplica1 = 14
cyclic_params1 = NO
rest_function1 = 1
[SELECTION]
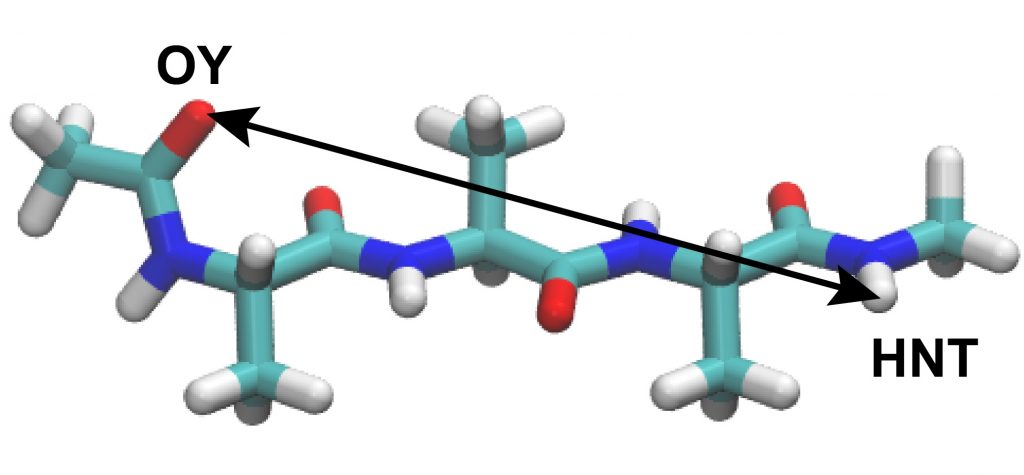
group1 = an:OY and resno:1 # restraint group 1
group2 = an:HNT and resno:3 # restraint group 2
[RESTRAINTS]
nfunctions = 1
function1 = DIST
constant1 = 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2
reference1 = 1.80 2.72 3.64 4.56 5.48 6.40 7.32 8.24 9.16 10.08 11.00 11.92 12.84 13.76
select_index1 = 1 2
This control file is almost same with the subsequent production run of the REUS simulation. Here, we specify exchange_period = 0 in the [REMD] section, which means that parameter exchange is not attempted during the simulation. In the [REMD], [SELECTION], and [RESTRAINTS] sections, we give REUS parameters. We apply restraints on the distance between the OY atom in Residue 1 and HNT atom in Residue 3. The target distance is ranging from 1.80 to 13.76 Å at the interval of 0.92 Å, and the force constant of the restraint potential is set to 1.2 kcal/mol/Å2 for all replicas. Note that 1.80 Å is nearly corresponding to the hydrogen bond distance, and 13.76 Å is a distance when the peptide forms a fully extended conformation.
Index of the restraint function to be used in REUS is specified in rest_function in [REMD] (red character above), and parameters to be exchanged in REUS are specified in constant and reference in [RESTRAINTS]. Note that the values in the same column in the constant and reference lines are packed into one “parameter set”, and they are exchanged between replicas. Specifically, if the user sets “constant1 = 1.2 1.4 1.6” and “reference1 = 1.80 2.72 3.64“, each replica has the parameter (1.2, 1.80) or (1.4, 2.72) or (1.6, 3.64).
In the [OUTPUT] section, we can see that there is a bracket “{}” in the filename. The replica number is automatically inserted into this bracket. For example, Replica1 yields eq_rep1.log, eq_rep1.dcd, and eq_rep1.rst. As for the other sections, we use common parameters to the conventional MD simulations (see Tutorial 3.2). The simulation is performed in the NVT ensemble at 300 K, and Bussi thermostat is used for the temperature control. The SHAKE/RATTLE and SETTLE algorithms are applied to the bonds including hydrogen and TIP3P water, respectively. We carry out 200 ps MD simulation for each replica.
If you are going to employ the NVT ensemble in the production run, you should NOT use the NPT ensemble at this stage. Otherwise, each replica has different volume in the production run.
Now, let’s execute spdyn. Since we have 14 replicas, the total number of MPI processors should be 14 × n, where n is the number of MPI processors employed for each replica. If you specify multiple OpenMP threads with “export OMP_NUM_THREADS=m“, the total number of CPU cores used for the simulation should be 14 × n × m. Please, specify the correct number of processors in your batch script, when you run the simulation. The following is an example command, in which 32 CPU cores are employed for one replica (448 CPU cores in total).
# Carry out the equilibration run
$ mpirun -np 112 -x OMP_NUM_THREADS=4 ../bin/spdyn INP > log
Number of MPI processors in each replica is automatically set to “total MPI processors ÷ number of replicas”. In addition, each replica keeps the rule of hybrid MPI/OpenMP parallelization for single MD simulation.
Let’s check the peptide conformation in the trajectory of each replica by using VMD. We can see that the conformation in Replica1 (short distance restraint) is compact, while it is extended in Replica 14 (long distance restraint), indicating that the equilibration runs with 14 individual restrains were correctly done.
# for Replica 1
$ vmd ../1_setup/wbox.pdb -psf ../1_setup/wbox.psf -dcd eq_rep1.dcd
# for Replica 14
$ vmd ../1_setup/wbox.pdb -psf ../1_setup/wbox.psf -dcd eq_rep14.dcd
4. Production run
We carry out a production run of the REUS simulation. Let us see the control file. The following shows important options.
# Change directory for production run
$ cd ../4_production
# View the control file
$ less INP
[INPUT]
topfile = ../1_setup/top_all36_prot.rtf # topology file
parfile = ../1_setup/par_all36m_prot.prm # parameter file
strfile = ../1_setup/toppar_water_ions.str # stream file
psffile = ../1_setup/wbox.psf # protein structure file
pdbfile = ../1_setup/wbox.pdb # PDB file
rstfile = ../3_equilibrate/eq_rep{}.rst # restart file
[OUTPUT]
logfile = prod_rep{}.log # log file of each replica
dcdfile = prod_rep{}.dcd # DCD trajectory file
rstfile = prod_rep{}.rst # restart file
remfile = prod_rep{}.rem # parameter index file
[REMD]
dimension = 1
exchange_period = 2000
type1 = RESTRAINT
nreplica1 = 14
cyclic_params1 = NO
rest_function1 = 1
[DYNAMICS]
integrator = VRES # RESPA integrator
nsteps = 4000000 # MD steps in one replica
timestep = 0.0025 # timestep (ps)
eneout_period = 200 # energy output period
crdout_period = 200 # coordinates output period
rstout_period = 4000000 # restart output period
nbupdate_period = 10 # nonbond update period
elec_long_period = 2 # period of reciprocal space calculation
thermostat_period = 10 # period of thermostat update
barostat_period = 10 # period of barostat update
The main difference from the previous pre-equilibration run is “exchange_period = 2000” in the [REMD] section. The parameter exchange is attempted every 2,000 steps. In the [OUTPUT] section, we define remfile, where the parameter index in each replica is output during the simulation. We perform 10-ns REUS simulation for each replica (10 ns × 14 replicas = 140 ns in total). Coordinates are output every 200 steps. Like the previous equilibration run, we execute spdyn.
# Perform production run
$ mpirun -np 112 -x OMP_NUM_THREADS=4 ../bin/spdyn INP > log
In the log file, information about replica-exchange attempt is output at every exchange_period step. For example, at 154,000 step you can see the following information (detailed results might be different from yours):
REMD> Step: 154000 Dimension: 1 ExchangePattern: 2
Replica ExchangeTrial AcceptanceRatio Before After
1 1 > 0 N 0 / 0 1 1
2 2 > 3 R 7 / 39 2 2
3 8 > 9 R 6 / 39 8 8
4 3 > 2 R 7 / 39 3 3
5 4 > 5 R 12 / 39 4 4
6 6 > 7 R 10 / 39 6 6
7 7 > 6 R 10 / 39 7 7
8 5 > 4 R 12 / 39 5 5
9 11 > 10 R 11 / 39 11 11
10 14 > 0 N 0 / 0 14 14
11 13 > 12 A 18 / 39 13 12
12 12 > 13 A 18 / 39 12 13
13 9 > 8 R 6 / 39 9 9
14 10 > 11 R 11 / 39 10 10
Parameter : 1 2 8 3 4 6 7 5 11 14 12 13 9 10
RepIDtoParmID: 1 2 8 3 4 6 7 5 11 14 12 13 9 10
ParmIDtoRepID: 1 2 4 5 8 6 7 3 13 14 9 11 12 10
In this case, the red line tells us that Replica 11 had Parameter 13 (k = 1.2, r = 12.84) before 154,000 step, and the exchange to Parameter 12 (k = 1.2, r = 11.92) was accepted (A) at 154,000 step, and then it has Parameter 12 after 154,000 step. The acceptance ratio between the parameter pairs 12 and 13 at 154,000 step is 18/39 (=46.2%). The blue line tells us that Replica 5 had Parameter 4 (k = 1.2, r = 4.56), but the exchange to Parameter 5 (k = 1.2, r = 5.48) was rejected (R), and then it has still Parameter 4 after 154,000 step. The green lines tell us that Replicas 1 and 10 have Parameters 1 and 14, respectively, but they have no neighboring pairs. Thus, replica exchange was not attempted (N) at this step.
We can see that ExchangePattern is 2 at 154,000 step (orange). In the exchange pattern = 2, Parameters 2-3, Parameters 4-5, Parameters 6-7, …, and Parameters 12-13 are defined as the neighboring pairs. On the other hand, in the exchange pattern = 1, Parameters 1-2, Parameters 3-4, Parameters 5-6, …, and Parameters 13-14 are defined as the neighboring pairs. In the one-dimensional REMD simulation, exchange pattern = 1 and 2 emerge alternatively at every exchange_period.
Purple lines summarize location of replica and parameter indexes. The RepIDtoParmID stands for the permutation function that converts Replica ID to Parameter ID. For example, in the 3rd column, 8 is written, which means that Replica 3 has Parameter 8. In the 9th column, 11 is written, indicating that Replica 9 has Parameter 11. The ParmIDtoRepID also stands for the permutation function that converts Parameter ID to Replica ID. For example, in the 3rd column, 4 is written, which means that Parameter 3 is located in Replica 4. In the 5th column, 8 is written, indicating that Parameter 5 is located in Replica 8. The Parameter line includes parameter index of each replica. In the case of REUS, this line is same with RepIDtoParmID line.
After the simulation is finished, we obtain trajectory and restart files from each replica.
If you want to restart the REUS simulation, do NOT change the order of the replica parameters in the control file before and after the restart, even if parameter exchange occurred during the simulation.
[RESTRAINTS]
constant1 = 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2
reference1 = 1.80 2.72 3.64 4.56 5.48 6.40 7.32 8.24 9.16 10.08 11.00 11.92 12.84 13.76
5 Analysis
In this section, we explain how to analyze REUS trajectories. All analyses are carried out in the 5_analysis directory.
# change directory
$ cd ../5_analysis
$ ls
1_convert_dcd 2_sort_dcd 3_calc_ratio 4_plot_index 5_calc_dist 6_calc_mbar 7_calc_pmf
5.1 Make converted trajectory files for analysis
Since the obtained “raw” DCD files contain many water molecules, it may take much time to analyze them. In order to save time, we first remove all water molecules from the trajectory files, and create new DCD files by using crd_convert. In addition, we superimpose the peptide structure of each snapshot onto the initial structure. Sample control file for crd_convert is already prepared in the 1_dcd_convert directory. For this purpose, we need 14 control files in total. These files were created with a script in the “script” directory.
# Change directory
$ cd 1_convert_dcd
$ ls
INP1 INP11 INP13 INP2 INP4 INP6 INP8 run.csh
INP10 INP12 INP14 INP3 INP5 INP7 INP9 script
# Run crd_convert for each
$ ../../bin/crd_convert INP1 > log1
$ ../../bin/crd_convert INP2 > log2
:
$ ../../bin/crd_convert INP14 > log14
# check the converted trajectory file by VMD
$ vmd replica1.pdb -dcd replica1.dcd
5.2 Sort coordinates in DCD files by parameters
In the raw DCD files generated from the REUS simulation, coordinates at different conditions (or replica parameters) are mixed. In order to sort the coordinates by replica parameters, we use remd_convert. Sorting is done based on the information in the remfile. In the 2_sort_dcd directory, sample control file is already prepared.
# Change directory
$ cd ../2_sort_dcd
# View the control file
$ less INP
[INPUT]
reffile = ../1_convert_dcd/replica1.pdb # PDB file
dcdfile = ../1_convert_dcd/replica{}.dcd # DCD file
remfile = ../../4_production/prod_rep{}.rem # REMD parameter ID file
logfile = ../../4_production/prod_rep{}.log # REMD energy log file
[OUTPUT]
pdbfile = param.pdb # PDB file
trjfile = param{}.dcd # trajectory file
logfile = param{}.log # REMD energy log file
[SELECTION]
group1 = all # selection group 1
[FITTING]
fitting_method = NO # method
[OPTION]
convert_type = PARAMETER # (REPLICA/PARAMETER)
convert_ids = # selected index (empty = all)
num_replicas = 14 # total number of replicas
nsteps = 4000000 # nsteps in [DYNAMICS]
exchange_period = 2000 # exchange_period in [REMD]
crdout_period = 200 # crdout_period in [DYNAMICS]
eneout_period = 200 # eneout_period in [DYNAMICS]
trjout_format = DCD # (PDB/DCD)
trjout_type = COOR+BOX # (COOR/COOR+BOX)
trjout_atom = 1 # atom group
centering = NO # shift center of mass
pbc_correct = NO # (NO/MOLECULE)
In the [INPUT] section, we specify dcdfile and PDB file obtained in the previous subsection. This is because input dcdfile should contain the same number and order of atoms with reffile. Of course, you can specify the original DCD and PDB files (../../4_production/step4_rep{}.dcd and ../../1_setup/wbox.pdb) for dcdfile and reffile, but it takes a very long time for trajectory convert, because they contain many water molecules. In the [OPTION] section, convert_type = PARAMETER is used to sort coordinates by replica parameters. Here, nsteps, exchange_period, crdout_period, and eneout_period must be idential to those used in the REUS simulation. We run remd_convert by the following command, and then obtain the sorted trajectory files.
# Run remd_convert
$ ../../bin/remd_convert INP > log
# Check the sorted dcd file for Parameter 1 (r = 1.80)
$ vmd param.pdb -dcd param1.dcd
5.3 Calculate the acceptance ratio
Acceptance ratio of the parameter exchange is one of the important factors that determine an efficiency of REMD simulations. The acceptance ratio is displayed in the log file as mentioned above, and we examine the data at the last step. Here, we show an example command to collect and summarize the data. Note that the acceptance ratio of “A to B” is identical to “B to A”, and we calculate only “A to B”. The averaged acceptance ratio was ~0.25, indicating that sufficient replica exchange was realized in the simulation.
# Change directory
$ cd ../3_calc_ratio
# Get the acceptance ratio between neighboring parameter pairs
$ grep ' 1 > 2' ../../4_production/log | tail -1 > log
$ grep ' 2 > 3' ../../4_production/log | tail -1 >> log
$ grep ' 3 > 4' ../../4_production/log | tail -1 >> log
:
$ grep '13 > 14' ../../4_production/log | tail -1 >> log
# Show the results
$ awk '{print $2,$3,$4,$6/$8}' log
1 > 2 0.343
2 > 3 0.22
3 > 4 0.116
4 > 5 0.162
5 > 6 0.269
6 > 7 0.225
7 > 8 0.178
8 > 9 0.172
9 > 10 0.261
10 > 11 0.267
11 > 12 0.268
12 > 13 0.326
13 > 14 0.427
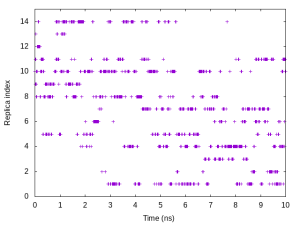
5.4 Plot the time courses of replica index and target distance
To examine a random walk of the system in replica space, we analyze the time courses of the replica index. We need to plot one column in the “ParmIDtoRepID:” lines in log onto the time axis. The following commands are example to plot the replica index that has Parameter 10 (k = 1.2 kcal/mol/Å2 and r = 10.08Å):
# Change directory
$ cd ../4_plot_index
# Create log file that contains replica index
$ grep "ParmIDtoRepID:" ../../4_production/log > index.log
# Plot the time courses of replica index that has Parameter 10
$ gnuplot
gnuplot> set xlabel 'Time (ns)'
gnuplot> set ylabel 'Replica index'
gnuplot> unset key
gnuplot> plot [][0:15]'index.log' u ($0/200):11
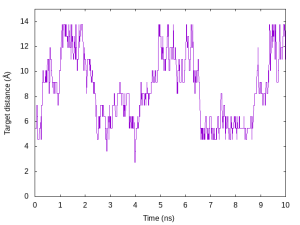
We also plot the time courses of the target distance of the restraint function in one replica. In the log file, parameter index is output in the “Parameter :” lines instead of the target distance. So, we should convert the index to the actual distance value. Here, we use awk script (./script/convert.awk) to convert them. The following commands are example to plot the target distance in Replica 5.
# Create log file that contains target distance
$ grep "Parameter :" ../../4_production/log > param.log
$ awk -f ./script/convert.awk param.log > dist.log
# Plot the time courses of target distance in Replica 5
$ gnuplot
gnuplot> set xlabel 'Time (ns)'
gnuplot> set ylabel 'Target distance (Å)'
gnuplot> unset key
gnuplot> plot [][0:15]'dist.log' u ($0/200):5 with lines
5.5 Analyze the end-to-end distance of (Ala)3
In this subsection, we calculate the end-to-end distance of the (Ala)3 by using trj_analysis. In the 5_calc_dist directory, we have two directories: replica and parameter. The former is used to analyze the original DCD files (../1_convert_dcd/replica{}.dcd), and the latter is used to analyze the sorted DCD files (../2_sort_dcd/param{}.dcd).
# Change directory
$ cd ../5_calc_dist
$ ls
parameter replica
First, let us calculate the end-to-end distance of the peptide in the original DCD file. Sample control files are already contained in the directory. Again, we have to prepare 14 individual control files to analyze each replica.
# Change directory
$ cd replica
$ ls
INP1 INP11 INP13 INP2 INP4 INP6 INP8 run.csh
INP10 INP12 INP14 INP3 INP5 INP7 INP9 script
# Check one control file
$ less INP1
[INPUT]
reffile = ../../1_convert_dcd/replica1.pdb # PDB file
[OUTPUT]
disfile = replica1.dis # distance file
[TRAJECTORY]
trjfile1 = ../../1_convert_dcd/replica1.dcd # trajectory file
md_step1 = 4000000 # number of MD steps
mdout_period1 = 200 # MD output period
ana_period1 = 200 # analysis period
repeat1 = 1
trj_format = DCD # (PDB/DCD)
trj_type = COOR+BOX # (COOR/COOR+BOX)
trj_natom = 0 # (0:uses reference PDB atom count)
[OPTION]
check_only = NO
distance1 = PROA:1:ALA:OY PROA:3:ALA:HNT
INP1 is used to analyze ../../1_convert_dcd/replica1.dcd. Because the DCD file should contain the same number and order of atoms with the input PDB file, we specify ../../1_convert_dcd/replica1.pdb as reffile. Here, we compute the distance between OY atom in Ala1 and HNT atom in Ala3. After running trj_analysis for INP1 to INP14, we obtain replica1.dis to replica14.dis. We plot the time courses of the distance in Replica 5 below as an example.
# Run trj_analysis to analyze the distance
# this is done for 1 to 14 (./run.csh is available for scripting)
$ ../../../bin/trj_analysis INP1 > log1
$ ../../../bin/trj_analysis INP2 > log2
:
$ ../../../bin/trj_analysis INP14 > log14
# Plot the time courses of the distance in Replica 5
$ gnuplot
gnuplot> set xlabel 'Time (ns)'
gnuplot> set ylabel 'Distance (Å)'
gnuplot> unset key
gnuplot> plot [][0:15]'replica5.dis' u ($0/2000):2 with lines
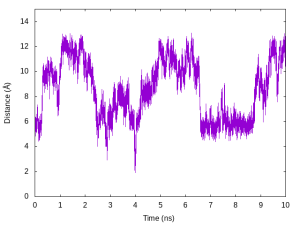
Let us compare the results with the above plot (changes in the target distance in Replica 5). We can see that the distance increases as the target distance increases, and vice versa.
Then, let us calculate the end-to-end distance in the sorted DCD files. Sample control files are already contained in the ./5_calc_dist/parameter directory. Again, we should prepare 14 individual control files to analyze each DCD file. After running trj_analysis, we obtain parameter1.dis to parameter14.dis as the output files. We plot the histogram of the end-to-end distance obtained in each restraint condition by using gnuplot. There are 20,000 distance data in each file, and we set the bin width to be 0.05Å.
# Change directory
$ cd ../parameter
$ ls
INP1 INP11 INP13 INP2 INP4 INP6 INP8 run.csh
INP10 INP12 INP14 INP3 INP5 INP7 INP9 script
# Run trj_analysis to analyze the distance
$ ../../../bin/trj_analysis INP1 > log1
$ ../../../bin/trj_analysis INP2 > log2
:
$ ../../../bin/trj_analysis INP14 > log14
# Plot the histogram of the end-to-end distance
$ gnuplot
gnuplot> set key outside
gnuplot> set xlabel 'Distance (Å)'
gnuplot> set ylabel 'Probability'
gnuplot> binwidth=0.05
gnuplot> bin(x,width)=width*floor(x/width)
gnuplot> ndata=20000
gnuplot> plot for [k=1:14] 'parameter'.k.'.dis' u (bin($2,binwidth)):(1.0/ndata) w l t 'Parameter'.k smooth freq
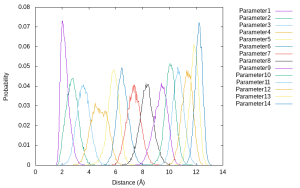
The results show that there is enough overlap between neighboring umbrella windows along the reaction coordinates. These overlaps are important to obtain reliable free energy profile in reweighting techniques such as WHAM.
5.6 Free energy calculation using MBAR
We calculate the free energy profile of the (Ala)3 as a function of the end-to-end distance by using MBAR. Here, we use mbar_analysis of the GENESIS analysis tool set. Let us move to the 6_calc_mbar directory, and see the sample control file:
# change directory
$ cd ../../6_calc_mbar
$ less INP
[INPUT]
cvfile = ../5_calc_dist/parameter/parameter{}.dis # Collective variable file
[OUTPUT]
fenefile = output.fene # free energy file
weightfile = output{}.weight # weight file
[MBAR]
nreplica = 14
input_type = CV
dimension = 1
nblocks = 1
temperature = 300
target_temperature = 300
rest_function1 = 1
[RESTRAINTS]
constant1 = 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2
reference1 = 1.80 2.72 3.64 4.56 5.48 6.40 7.32 \
8.24 9.16 10.08 11.00 11.92 12.84 13.76
is_periodic1 = NO
In the [INPUT] section, we specify the output file generated from the distance analysis for the sorted DCD files (see the previous subsection). In the [MBAR] section, we set the number of the dimension of the reaction coordinates, the temperature used in the REUS simulation, and so on. For the [RESTRAINTS] section, we give the same information used in the REUS control file.
# perform mbar
$ ../../bin/mbar_analysis INP > log
This produces “output.fene” file containing the evaluated relative free energies and 14 “output*.weight” files containing the weights of each snapshot for each replica. For example, output1.weight is as follows:
$ less output1.weight
1 1.4097966267636603E-007
2 1.5805208264591707E-007
3 1.5488288652400664E-007
4 1.4596171898407404E-007
5 1.6880812728502563E-007
6 1.8985319931591140E-007
7 1.4525835770429576E-007
:
:
19997 1.4189940392490833E-007
19998 1.6620382169517072E-007
19999 1.4418046201344524E-007
20000 1.5028161473380590E-007
5.7 Calculating PMF of distance distribution
The final step of this tutorial is to use the calculated distances in 5.5 and weight files from MBAR analysis (5.6) to calculate PMF of end to end distance distribution in (Ala)3. Here, we use pmf_analysis of the GENESIS analysis tool set. Let us move to the 7_calc_pmf directory, and see the sample control file:
# change directory
$ cd ../7_calc_pmf
$ less INP
[INPUT]
cvfile = ../5_calc_dist/parameter/parameter{}.dis # collective variable file
weightfile = ../6_calc_mbar/output{}.weight # weight file
[OUTPUT]
pmffile = dist.pmf # potential of mean force file
[OPTION]
nreplica = 14 # number of replicas
dimension = 1 # dimension of cv space
temperature = 300
grids1 = 0.0 15.0 601 # (min max num_of_bins)
band_width1 = 0.25 # sigma of gaussian kernel
# should be comparable or smaller than the grid size
# (pmf_analysis creates histogram by accumulating gaussians)
is_periodic1 = NO # periodicity of cv1
# perform pmf
$ ../../bin/pmf_analysis INP > log
# plot the PMF of end to end distance
$ gnuplot
gnuplot> set xlabel 'Distance (Å)'
gnuplot> set ylabel 'Potential of mean force (kcal/mol)'
gnuplot> plot [0:13][0:10]'dist.pmf' u 1:3 w lines
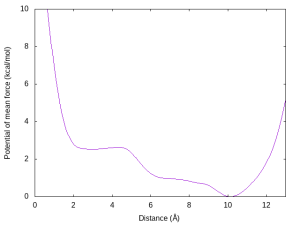
We can see that there is the global energy minimum around r = 10.1 Å and a local energy minimum around r = 2.8 Å. The latter corresponds to the α-helix conformation, where the hydrogen bond between OY and HNT is formed. These results suggest that in water the (Ala)3 tends to form an extended conformation rather than α-helix. Note that this 10-ns simulation might be still short to get a reliable free energy profile. The users are strongly recommended to check the convergence of the free energy profile. If you extend the simulation time, and the free energy profile was largely changed, your simulation might be still short (or structure sampling is not sufficient) (for details, see ref [4]).
References
- Y. Sugita, A. Kitao, and Y. Okamoto, J. Chem. Phys., 113, 6042 (2000).
- H. Hukunishi, O. Watanabe, and S. Takada, J. Chem. Phys., 116, 9058 (2002).
- S. Park, T. Kim, and W. Im, Phys. Rev. Lett., 108, 108102 (2012).
- H. Oshima, S. Re, and Y. Sugita, J. Chem. Theory Comput., 15, 10, 5199 (2019).
Written by Takaharu Mori@RIKEN Theoretical molecular science laboratory
April 26, 2022
